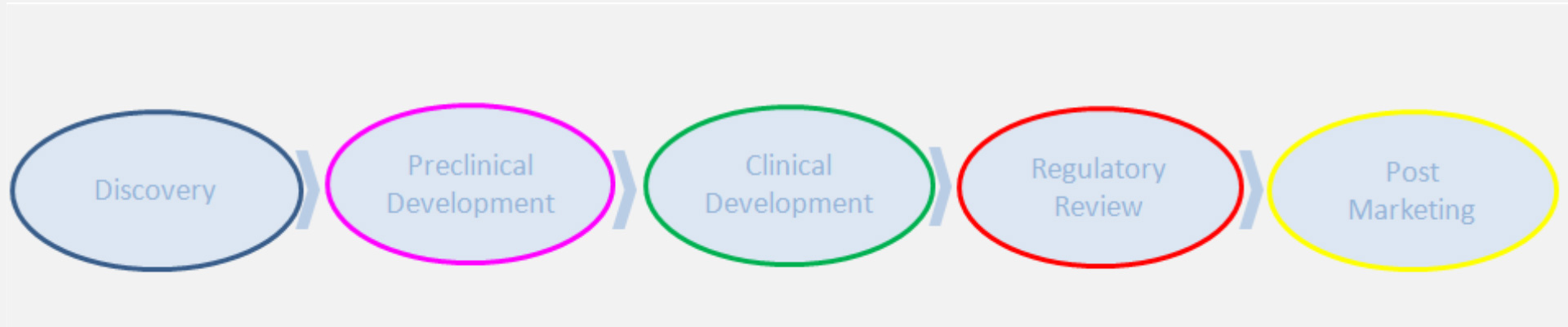

Clinical Development
Advising, designing, writing or reviewing:
- Clinical development plans
- Biomarker strategy
- Clinical protocol synopses and protocols
- Clinical reports/publications
- Clinical expert reports
- Investigator brochures
- Ad hoc clinical documents for FDA/EMA submission (eg: pre-IND, IND)
Clinical Safety
Reviewing or Advising:
- Individual AE/SAE reports
- Study safety results interpretation
- Periodic regulatory safety updates

Regulatory Strategy
Reviewing or Advising:
- Ad hoc interaction with regulatory authorities (eg: FDA, EMA)
- Conventional or Accelerated submission
- Region or country selection
- Phase 1B/2 objectives definition

Project Evaluation
Reviewing or Advising:
- Preclinical package to enter in human
- Early clinical data to progress to the next step
- Preclinical or clinical data for in-licencing program
Problem Solutions
Reviewing or Advising:
- Design of functions for early clinical development organisations
- Site selection
- CRO selection

Training and Lecturing
Reviewing or Advising:
- Preparing presentations for internal, external or scientific meetings
- Discussing protocols with potential investigators
- Training and lecturing on topics especially related to clinical drug development, study design, good clinical practices (GCP), drug safety and pharmacovigilance.

Literature survey
- Internal decision making purposes
- Background for publications
- Medical information material
